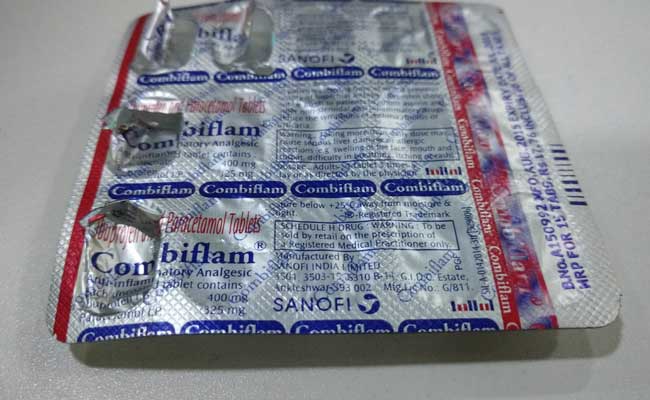
Painkiller Combiflam was withdrawn off its vast stockpile in the country after the Central Drugs Standard Control Organisation (CDSCO), found the stock second-rated.
Some batches of Combiflam were found to be “not of standard quality” as they failed disintegration tests, said the CDSCO in notices posted on its website.
Disintegration tests are used to test the time it takes for tablets and capsules to break down inside the body, and are used as a quality-assurance measure in pharmaceuticals.
Combiflam is an amalgamation of paracetamol and ibuprofen, and is one of French company Sanofi’s five biggest brands in India, according to the company’s latest available annual report dated March, 2015.
The drug batches adduced by the CDSCO were manufactured in June, 2015 and July, 2015, and bore expiry dates of May, 2018 and June, 2018, according to the notices.
“In the case of Combiflam, though the disintegration time was divulged, doctors and patients can be assured that there is no impact on the safety and efficacy of the product,” a Sanofi spokeswoman said in an email to news agency Reuters. Sanofi India’s shares were down by 2 percent in early trade on Thursday.
By Isha Kulkarni with inputs from NDTV
Tags: CDSCO Combiflam india painkiller Sanofi